Purine and Pyrimidine Structures
The pyrimidine bases have a 6‐membered ring with two nitrogens and four carbons.

![]()
![]()
![]()
The purine bases have a 9‐membered double‐ring system with four nitrogens and five carbons.

Although both purine and pyrimidine rings have one 6‐membered component with two nitrogens and four carbons, the purines and pyrimidnes are not related metabolically. Distinct pathways for purine biosynthesis and degradation and for pyrimidine biosynthesis and degradation, exist in all organisms.
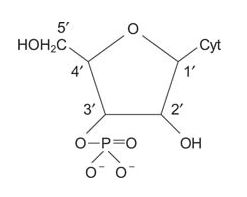
The combination of a 5‐membered carbohydrate ring and a purine or pyrimidine is called a nucleoside. The rings are numbered as shown in the following figure. The two rings of a nucleoside or nucleotide must be distinguished from each other, so the positions of the sugar carbons are denoted with a '(prime) notation. If one or more phosphates exist on the carbohydrate, the combination is called a nucleotide. For example, ATP is a nucleotide.
- Deoxyguanosine is a nucleoside as is 2′‐O‐methyladenosine.

- 3′‐cytidine monophosphate is a nucleotide.