Triacylglycerols (fats and oils) store the majority of the energy in most animals and plants. Fats such as beef tallow remain solid or semisolid at room temperature while oils such as olive oil or corn oil are liquid at that temperature. Oils solidify only at lower temperatures—in a refrigerator, for example. The different kinds of fatty acids found in the side chains of the triacylglycerol cause the different melting temperatures. The fatty acids of oils contain more double bonds than do those of fats.
These molecules make good energy‐storing units because their oxidation releases more energy than the oxidation of carbohydrates or amino acids. The caloric density of triacylglyerols is about 9 kilocalories per gram, compared to 5 kilocalories per gram for the latter biomolecules.
Essential fatty acids are precursors to membrane lipids and to compounds that serve as intercellular signals in animals.
The caloric density of lipids is due to the side chain carbons of fats being more reduced (hydrogen‐rich) than the side chain carbons of carbohydrates, for example: ![]()
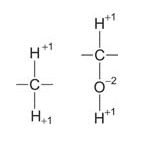
Fat, oxidation state = −2 → Carbohydrate, oxidation state = 0
Oxidation of the carbon found in fatty acids to carbon dioxide involves a change in oxidation number from −2 to +4, while the oxidation of the carbon of carbohydrate involves a change from 0 to +4. The greater change in oxidation number means that the oxidation of fat releases more energy. (This is a general principle; for example, burning methane, CH 4, releases more heat than burning methanol, CH 2OH). On the other hand, while amino acids and carbohydrates can oxidize anaerobically (without added oxygen), fats can oxidize only aerobically. Many cultures have used this characteristic to preserve foods in animal fat. The fat prevents the growth of oxygen‐requiring molds and bacteria.