In humans, excess amino groups are detoxified by conversion to urea. Ammonia in high concentrations is toxic to all organisms. For example, humans with kidney failure die because the level of ammonia increases in the bloodstream. Organisms that live in water, including fish and microorganisms, simply eliminate ammonia directly and allow it to be diluted by their environment. Terrestrial organisms, on the other hand, convert the ammonium groups to other compounds that are less toxic. Birds and reptiles synthesize uric acid as the primary nitrogen excretion compound. The white crystals of uric acid constitute the unsightly part of bird droppings; the poor solubility of uric acid makes the results hard to remove from a car or a statue in a park, for example. Mammals synthesize urea, ultimately from carbonate and ammonium ions.

Figure 1
In mammals, muscle breakdown or excess protein intake results in an imbalance between the fates of the carbon chains and the amino nitrogen. Unlike fat (lipid storage) or glycogen (carbohydrate storage), excess amino acids are not stored in polymeric form for later utilization. The carbon chains of amino acids are generally metabolized into tricarboxylic acid (TCA) cycle intermediates, although it is also possible to make ketone bodies such as acetoacetate from some. Conversion to TCA intermediates is easy to see in some instances. For example, alanine is directly transaminated to pyruvate.
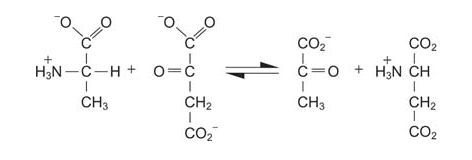
![]()
More complex processes convert other amino acids, especially the branched chained ones.
The nutritional consequences of an excess protein diet are the same as those of an excess carbohydrate or excess fat diet: lipid biosynthesis and fat deposition. Additionally, the protein amino groups must be detoxified and eliminated. The nutritional consequences of a diet lacking complete protein—that is, one that doesn't supply the essential amino acids in the proportions needed for synthesis of proteins and neurotransmitters—also include excess ammonia generation. In this case, muscle proteins are degraded to supply enough of the limiting essential amino acid. The other amino acids are broken down, with the carbon chains metabolized into carbohydrates (and, potentially lipid). The leftover amino groups must then be eliminated as urea.
Biochemistry of the urea cycle
First the ammonium ions must be activated through conversion to carbamoyl phosphate by the mitochondrial form of carbamoyl phosphate synthetase.


![]()
The entry of activated ammonia into the urea cycle occurs by the ornithine transcarbamoylase reaction where the carbamoyl group is transferred to the side chain amino group of the non‐protein amino acid, ornithine. Ornithine has five carbons; its carbon chain therefore has the same length as that of arginine. The product of the ornithine transcarbamoylase reaction is the amino acid citrulline.

Figure 2
The addition of the second ammonia to the backbone involves the joining of the carbamoyl group of citrulline to the χ‐amino group of aspartate, leading to a complex compound, arginosuccinate. ATP is hydrolyzed in this step.

![]()
The next step is cleavage of arginosuccinate to arginine and fumarate by the enzyme arginosuccinate lyase. Lyases cleave bonds with the creation of a double bond in one of the products. In this case, the double bond is the carbon‐carbon double bond of fumarate.

![]()
The final step is the release of urea by the enzyme arginase, which regenerates ornithine.
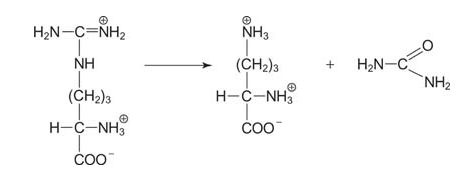
![]()
The other part of the urea cycle that has occurred is the conversion of the carbons of aspartate to fumarate. The fumarate is recycled back to oxaloacetate through TCA cycle reactions in the mitochondrion. Transamination with glutamate regenerates aspartate. The glutamate comes from the glutamate dehydrogenase reaction.
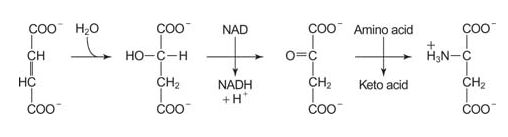
![]()
The urea is excreted through the kidneys and broken down to carbon dioxide and ammonia by plants and microorganisms. The enzyme urease causes this conversion.