Fatty Acyl‐CoA: β‐Oxidation Helical Scheme
In the mitochondrion, even‐numbered fatty acyl‐CoA is broken into acetyl‐CoA units starting from the carboxyl end. The first reaction is dehydrogenation by an FAD‐dependent dehydrogenase to form an enoyl‐CoA.
Reaction 1:
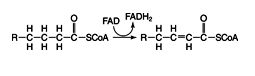
This reaction absolutely depends on the fatty acids being activated by CoA. This reaction is very similar to the succinate dehydrogenase step of the Krebs cycle.
Succinate dehydrogenase: ![]()
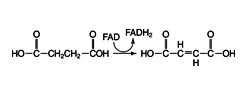
The enoyl‐CoA is then a substrate for the addition of water across the carbon‐carbon double bond. This results in a β‐hydroxy‐acyl‐CoA compound, because the OH of water is added to the carbon further away from the carboxyl group:
Reaction 2: ![]()
Again, this type of reaction occurs in the Krebs cycle, with the addition of water to fumarate to make malate.
fumarase: ![]()

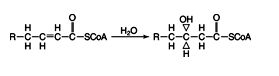
The hydrogens of the β‐hydroxy group are removed in a dehydrogenation reaction, this time using NAD as the electron acceptor.
Reaction 3: ![]()

This also occurs in the Krebs cycle, as in the dehydrogenation of malate to oxaloacetate.
malate dehydrogenase: ![]()

The final step in the removal of two carbons from the fatty acid is the thiolytic cleavage to release acetyl‐CoA. The term “thiolytic” refers to the use of Coenzyme A to bond with the carbonyl carbon of the β‐keto acid.
Reaction 4: ![]()
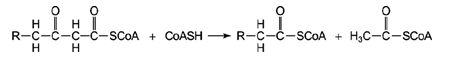
This step leaves two cleavage products. The first, derived from the two carbons at the carboxyl end of the fatty acid, is acetyl‐CoA, which can be further metabolized in the TCA cycle. The second cleavage product is a shorter fatty acyl‐CoA. Thus, for example, the initial step of digesting a fatty acid with 16 carbons is an acyl‐CoA molecule where the acyl group has 14 carbons and a molecule of acetyl‐CoA. The β‐oxidation scheme may be used to accommodate unsaturated fatty acids also. The reactions occur as described previously for the saturated portions of the molecule. Where a trans carbon‐carbon double bond occurs between the χ‐ and β‐carbons of the acyl‐CoA, the accommodation is fairly simple: reaction 1 isn't needed. Where the double bonds are in the cis configuration, or are between the β and γ carbons, isomerase enzymes change the location of the double bonds to make recognizable substrates for β‐oxidation.
Acetyl‐CoA from fatty acid oxidation enters the TCA cycle in the same way as does acetyl‐CoA derived from glucose: addition to oxaloacetate to make citrate. This can cause complications if an individual is metabolizing only fat, because the efficient metabolism of fat requires a supply of TCA‐cycle intermediates, especially dicarboxylic acids, which can't (usually) be made from fatty acids. These intermediates must be supplied by the metabolism of carbohydrates, or more often, amino acids derived from muscle tissue.