Ammonia is toxic at high concentrations, even though ammonium ion, NH 4 + is an intermediate in many reactions. For its utilization, ammonia must be incorporated into organic forms, transferred, and then incorporated into other compounds, for example, amino acids and nucleotides. The amino acids glutamine and glutamate and the compound carbamoyl phosphate are the key intermediates of nitrogen assimilation, leading to different classes of compounds.
Glutamate
Glutamate dehydrogenase has a relatively high K m for ammonia. The high Michaelis constant, K m, means that this system operates most effectively when ammonia is relatively abundant. Incorporation of ammonia uses reducing equivalents.
- glutamate dehydrogenase:

Glutamine
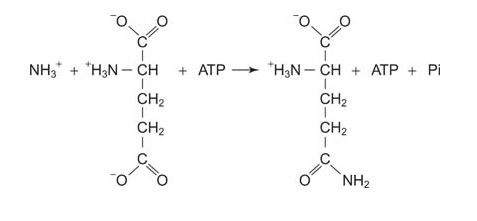
Glutamine synthetase requires ATP energy for ammonia incorporation, using glutamate as an acceptor.
![]()
Glutamate from glutamine
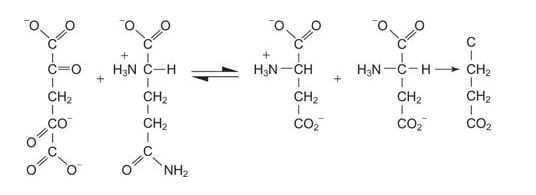
Glutamine can be a precursor for the synthesis of glutamate, with the reaction of glutamate synthase, also known as GOGAT (glutamine: 2‐oxyglutarate aminotransferase).
![]()
The preceding reactions indicate that two ways exist of making glutamate:
glutamate dehydrogenase
or
glutamine synthetase + glutamate synthase.
The relative activity of these two pathways depends on the metabolic conditions of the cell. Ammonia is lost from the cell relatively easily. When reduced nitrogen is relatively abundant, glutamate dehydrogenase is more active, because its K m for ammonia is higher than is that of glutamine synthetase. This may be expected to occur in bacteria growing in a medium containing a high concentration of ammonium salts. On the other hand, when ammonia is relatively non‐abundant (for example, when it is supplied by fixation), the pathway involving glutamine synthetase and glutamate synthase is more active. This second pathway uses ATP energy to move ammonia to an amino acid that cannot leak out of the cell.