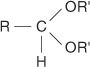
acetal the product formed by the reaction of an aldehyde with an alcohol. The general structure of an acetal is:
achiral the opposite of chiral; also called nonchiral. An achiral molecule can be superimposed on its mirror image.
acid see Brønsted-Lowry theory of acids and bases, and Lewis theory of acids and bases.
acid-base reaction a neutralization reaction in which the products are a salt and water.
activated complex molecules at an unstable intermediate stage in a reaction.
activating group a group that increases the rate of electrophilic aromatic substitution when bonded to an aromatic ring.
activation energy the energy that must be supplied to chemicals to initiate a reaction; the difference in potential energy between the ground state and the transition state of molecules. Molecules of reactants must have this amount of energy to proceed to the product state.
acyl group a group with the following structure, where R can be either an alkyl or aryl group.
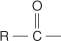
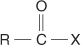
acyl halide a compound with the general structural formula:
acylation a reaction in which an acyl group is added to a molecule.
acylium ion the resonance stabilized cation:

addition a reaction that produces a new compound by combining all of the elements of the original reactants.
addition elimination mechanism the two-stage mechanism by which nucleophilic aromatic substitution occurs. In the first stage, addition of the nucleophile to the carbon bearing the leaving group occurs. An elimination follows in which the leaving group is expelled.
adduct the product of an addition reaction.
alcohol an organic chemical that contains an —OH group.
aldehyde an organic chemical that contains a —CHO group.
alicyclic compound an aliphatic cyclic hydrocarbon, which means that a compound contains a ring but not an aromatic ring.
aliphatic compound a straight- or branched-chain hydrocarbon; an alkane, alkene, or alkyne.
alkane a hydrocarbon that contains only single covalent bonds. The alkane general formula is CnH2n + 2.
alkene a hydrocarbon that contains a carbon-carbon double bond. The alkene general formula is CnH2n.
alkoxide ion an anion formed by removing a proton from an alcohol; the RO− ion.
alkoxy free radical a free radical formed by the homolytic cleavage of an alcohol —OH bond; the RO· free radical.
alkyl group an alkane molecule from which a hydrogen atom has been removed. Alkyl groups are abbreviated as "R" in structural formulas.
alkyl halide a hydrocarbon that contains a halogen substituent, such as fluorine, chlorine, bromine, or iodine.
alkyl-substituted cycloalkane a cyclic hydrocarbon to which one or more alkyl groups are bonded. (Compare with cycloalkyl alkane.)
alkylation a reaction in which an alkyl group is added to a molecule.
alkyne a hydrocarbon that contains a triple bond. The alkyne general formula is CnH2n − 2.
allyl group the H2C==CHCH2— group.
allylic carbocation the H2C==CHCH2+ ion.
analogue in organic chemistry, chemicals that are similar to each other, but not identical. For example, the hydrocarbons are all similar to each other, but an alkane is different from the alkenes and alkynes because of the types of bonds they contain. Therefore, an alkane and an alkene are analogues.
angle of rotation (α) in a polarimeter, the angle right or left in which plane-polarized light is turned after passing through an optically active compound in solution.
anion a negatively charged ion.
antibonding molecular orbital a molecular orbital that contains more energy than the atomic orbitals from which it was formed; in other words, an electron is less stable in an antibonding orbital than it is in its original atomic orbital.
anti-Markovnikov addition a reaction in which the hydrogen atom of a hydrogen halide bonds to the carbon of a double bond that is bonded to fewer hydrogen atoms. The addition takes place via a free-radical intermediate rather than a carbocation. (Compare with Markovnikov rule.)
arene an aromatic hydrocarbon.
aromatic compound a compound that possesses a closed-shell electron configuration as well as resonance. This type of compound obeys Hückel's rule.
aryl group a group produced by the removal of a proton from an aromatic molecule.
aryl halide a compound in which a halogen atom is attached to an aromatic ring.
atom the smallest amount of an element; a nucleus surrounded by electrons.
atomic mass (A) the sum of the weights of the protons and neutrons in an atom. (A proton and neutron each have a mass of 1 atomic mass unit.)
atomic number (Z) the number of protons or electrons in an atom.
atomic 1s orbital the spherical orbital nearest the nucleus of an atom.
atomic orbital a region in space around the nucleus of an atom where the probability of finding an electron is high.
atomic p orbital an hourglass-shaped orbital, oriented on x, y, and z axes in three-dimensional space.
atomic s orbital a spherical orbital.
Baeyer reagent Cold, dilute potassium permanganate, which is used to oxidize alkenes and alkynes.
base see Brønsted-Lowry theory of acids and bases, and Lewis theory of acids and bases.
benzenoid ring an aromatic ring with a benzene-like structure.
benzyl group the C6H5CH2 group.
benzyne an unstable intermediate that consists of a benzene ring with an additional bond that is created by the side-to-side overlap of sp2 orbitals on adjacent carbons of the ring.
bond angle the angle formed between two adjacent bonds on the same atom.
bond-dissociation energy the amount of energy needed to homolytically fracture a bond.
bond length the equilibrium distance between the nuclei of two atoms or groups that are bonded to each other.
bond strength see bond-dissociation energy.
bonding electron see valence electrons.
bonding molecular orbital the orbital formed by the overlap of adjacent atomic orbitals.
branched-chain alkane an alkane with alkyl groups bonded to the central carbon chain.
Brønsted-Lowry theory of acids and bases A Brønsted-Lowry acid is a compound capable of donating a proton (a hydrogen ion), and a Brønsted-Lowry base is capable of accepting a hydrogen ion. In neutralization, an acid donates a proton to a base, creating a conjugate acid and a conjugate base.
carbanion a carbon atom bearing a negative charge; a carbon anion.
carbene an electrically uncharged molecule that contains a carbon atom with only two single bonds and just six electrons in its valence shell.
carbenoid a chemical that resembles a carbene in its chemical reactions.
carbocation a carbon cation; a carbon atom bearing a positive charge (sometimes referred to as a "carbonium ion").
carbonyl group the 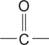 group.
group.
carboxylic acid the 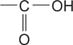 group.
group.
catalyst a substance that affects the rate of a reaction in which it participates; however, it is not altered or used up in the process.
cation a positively charged ion.
cationic polymerization occurs via a cation intermediate and is less efficient than free-radical polymerization.
chain reaction a reaction that, once started, produces sufficient energy to keep the reaction running. These reactions proceed by a series of steps, which produce intermediates, energy, and products.
chemical shift a position in an NMR spectrum, relative to TMS, at which a nucleus absorbs.
chiral describes a molecule that is not superimposable on its mirror image; like the relationship of a left hand to a right hand.
closed-shell electron configuration a stable electron configuration in which all of the electrons are located in the lowest energy orbitals available.
competing reactions two reactions that start with the same reactants but form different products.
concerted taking place at the same time without the formation of an intermediate.
condensation reaction a reaction in which two molecules join with the liberation of a small stable molecule.
conjugate acid the acid that results when a Brønsted-Lowry base accepts a hydrogen ion.
conjugate base the base that results when a Brønsted-Lowry acid loses a hydrogen ion.
conjugated double bonds carbon-carbon double bonds that are separated from one another by one single bond.
conjugation the overlapping in all directions of a series of p orbitals. This process usually occurs in a molecule with alternating double and single bonds.
conjugation energy see resonance energy.
coupling constant (J) the separation in frequency units between multiple peaks in one chemical shift. This separation results from spin-spin coupling.
covalent bond a bond formed by the sharing of electrons between atoms.
cyano group —C≡≡N the group.
cyanohydrin a compound with the general formula
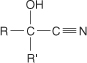
cyclization the formation of ring structures.
cycloaddition a reaction that forms a ring.
cycloalkane a ring hydrocarbon made up of carbon and hydrogen atoms joined by single bonds.
cycloalkyl alkane an alkane to which a ring structure is bonded.
cyclohydrocarbon an alkane, alkene, or alkyne formed in a ring structure rather than a straight or branched chain. The cyclohydrocarbon general formula is CnH2n (n must be a whole number of 3 or greater).
deactivating group a group that causes an aromatic ring to become less reactive toward electrophilic aromatic substitution.
debye unit (D) the unit of measure for a dipole moment. One debye equals 1.0 × 10−18 esu · cm. (See dipole moment.)
decarboxylation a reaction in which carbon dioxide is expelled from a carboxylic acid.
dehalogenation the elimination reaction in which two halogen atoms are removed from adjacent carbon atoms to form a double bond.
dehydration the elimination reaction in which water is removed from a molecule.
dehydrohalogenation the elimination reaction in which a hydrogen atom and a halogen atom are removed from a molecule to form a double bond.
delocalization the spreading of electron density or electrostatic charge across a molecule.
delocalization energy see resonance energy.
deprotonation the loss of a proton (hydrogen ion) from a molecule.
deshielding an effect in NMR spectroscopy that the movement of σ and π electrons within the molecule causes. Deshielding causes chemical shifts to appear at lower magnetic fields (downfield).
Diels-Alder reaction a cycloaddition reaction between a conjugated diene and an alkene that produces a 1,4-addition product.
diene an organic compound that contains two double bonds.
dienophile the alkene that adds to the diene in a Diels-Alder reaction.
dihalide a compound that contains two halogen atoms; also called a dihaloalkane.
diol a compound that contains two hydroxyl (—OH) groups; also called a dihydroxy alkane.
dipole moment a measure of the polarity of a molecule; it is the mathematical product of the charge in electrostatic units (esu) and the distance that separates the two charges in centimeters (cm). For example, substituted alkynes have dipole moments caused by differences in electronegativity between the triple-bonded and single-bonded carbon atoms.
distillation the separation of components of a liquid mixture based on differences in boiling points.
double bond a multiple bond composed of one σ bond and one π bond. Rotation is not possible around a double bond. Hydrocarbons that contain one double bond are alkenes, and hydrocarbons with two double bonds are dienes.
E1 an elimination reaction mechanism in which the slow step is a self-ionization of the molecule to form a carbocation. Thus, the rate-controlling step is unimolecular.
E2 an elimination reaction mechanism in which the rate-controlling step is the simultaneous removal of a proton from the molecule by a base, resulting in the creation of a double bond. The rate controlling step is bimolecular.
electron negatively charged particles of little weight that exist in quantized probability areas around the atomic nucleus.
electron affinity the amount of energy liberated when an electron is added to an atom in the gaseous state.
electronegativity the measure of an atom's ability to attract electrons toward itself in a covalent bond. The halogen fluorine is the most electronegative element.
electronegativity scale an arbitrary scale by which the electronegativity of individual atoms can be compared.
electrophile an "electron seeker;" an atom that seeks an electron to stabilize itself.
electrophilic addition a reaction in which the addition of an electrophile to an unsaturated molecule results in the formation of a saturated molecule.
electrostatic attraction the attraction of a positive ion for a negative ion.
element of unsaturation a π bond; a multiple bond or ring in a molecule.
enantiomer a stereoisomer that cannot be superimposed on its mirror image.
enantiomorphic pair in optically active molecules with more than one stereogenic center, the two structures that are mirror images of each other are enantiomorphic pairs.
energy of reaction the difference between the total energy content of the reactants and the total energy content of the products. The greater the energy of reaction, the more stable the products.
enol an unstable compound (for example, vinyl alcohol) in which a hydroxide group is attached to a carbon bearing a carbon-carbon double bond. These compounds tautomerize to form ketones, which are more stable.
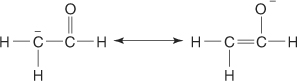
enolate ion the resonance stabilized ion formed when an aldehyde or ketone loses an α hydrogen.
epoxide a three-membered ring that contains oxygen.
ester the  functional group.
functional group.
ether an organic compound in which an oxygen atom is bonded to carbon atoms. The general formula is R—O—R′. Epoxyethane, an epoxide, is a cyclic ether.
free radical an atom or group that has a single unshared electron.
free-radical chain reaction a reaction that proceeds by a free-radical intermediate in a chain mechanism—a series of self-propagating, interconnected steps. (Compare with free-radical reaction.)
free-radical polymerization a polymerization initiated by a free radical.
free-radical reaction a reaction in which a covalent bond is formed by the union of two radicals. (Compare with free-radical chain reaction.)
functional group a set of bonded atoms that displays a specific molecular structure and chemical reactivity when bonded to a carbon atom in the place of a hydrogen atom.
Grignard reagent an organometallic reagent in which magnesium metal inserts between an alkyl group and a halogen; for example, CH3MgBr.
haloalkane an alkane that contains one or more halogen atoms; also called an alkyl halide.
halogen an electronegative, nonmetallic element in Group VII of the periodic table, including fluorine, chlorine, bromine, and iodine. Halogens are often represented in structural formulas by an "X."
halogenation a reaction in which halogen atoms are bonded to an alkene at the double bond.
halonium ion a halogen atom that bears a positive charge. This ion is highly unstable.
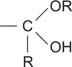
hemiacetal a functional group of the structure
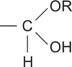
hemiketal a functional group of the structure
hertz a measure of a wave's frequency. A hertz equals the number of waves that passes a specific point per second.
hetero atom in organic chemistry, an atom other than carbon.
heterocyclic compound a class of cyclic compounds in which one of the ring atoms is not carbon; epoxyethane, for example.
heterogenic bond formation a type of bond formed by the overlap of orbitals on adjacent atoms. One orbital of the pair donates both of the electrons to the bond.
heterolytic cleavage the fracture of a bond in such a manner that one of the atoms receives both electrons. In reactions, this asymmetrical bond rupture generates carbocation and carbanion mechanism.
homologous series a set of compounds with common compositions; for example, the alkanes, the alkenes, and the alkynes.
homologue one of a series of compounds in which each member differs from the next by a constant unit.
homolytic cleavage the fracture of a bond in such a manner that both of the atoms receive one of the bond's electrons. This symmetrical bond rupture forms free radicals; in reactions, it generates free-radical mechanisms.
Hückel's rule a rule stating that a compound with 4n + 2 π electrons will have a closed shell electron configuration and will be aromatic.
hydration the addition of the elements of water to a molecule.
hydride shift the movement of a hydride ion, a hydrogen atom with a negative charge, to form a more inductively stabilized carbocation.
hydroboration the addition of boron hydride to a multiple bond.
hydroboration-oxidation the addition of borane (BH3) or an alkyl borane to an alkene and its subsequent oxidation to produce the anti-Markovnikov indirect addition of water.
hydrocarbon a molecule that contains exclusively carbon and hydrogen atoms. The central bond may be a single, double, or triple covalent bond, and it forms the backbone of the molecule.
hydrogenation the addition of hydrogen to a multiple bond.
hydrohalogenation a reaction in which a hydrogen atom and a halogen atom are added to a double bond to form a saturated compound.
hydrolyze to cleave a bond via the elements of water.
inductive effect the electron donating or electron withdrawing effect that is transmitted through σ bonds. It can also be defined as the ability of an alkyl group to "push" electrons away from itself. The inductive effect gives stability to carbocations and makes tertiary carbocations the most stable.
infrared spectroscopy a type of spectroscopy that provides structural information about a molecule, based on the molecule's interaction with energy from infrared light.
initiation step the first step in the mechanism of a reaction.
initiator a material capable of being easily fragmented into free radicals, which in turn initiate a free-radical reaction.
insertion placing between two atoms.
intermediate a species that forms in one step of a multistep mechanism; intermediates are unstable and cannot be isolated.
ion a charged atom; an atom that has either lost or gained electrons.
ionic bond a bond formed by the transfer of electrons between atoms, resulting in the formation of ions of opposite charge. The electrostatic attraction between these ions is the ionic bond.
ionization energy the energy needed to remove an electron from an atom.
isolated double bond a double bond that is more than one single bond away from another double bond in a diene.
isomers compounds that have the same molecular formula but different structural formulas.
IUPAC nomenclature a systematic method for naming molecules based on a series of rules developed by the International Union of Pure and Applied Chemistry. IUPAC nomenclature is not the only system in use, but it is the most common.
Kekulé structure the structure for benzene in which there are three alternating double and single bonds in a six-membered ring of carbon atoms.
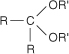
ketal the product formed by the reaction of a ketone with an alcohol. The general structure of a ketal is:
keto-enol tautomerization the process by which an enol equilibrates with its corresponding aldehyde or ketone.
ketone a compound in which an oxygen atom is bonded via a double bond to a carbon atom, which is itself bonded to two more carbon atoms.
kinetics the study of reaction rates.
leaving group the negatively charged group that departs from a molecule, which is undergoing a nucleophilic substitution reaction.
Lewis theory of acids and bases a Lewis acid is a compound capable of accepting an electron pair, and a Lewis base is capable of donating an electron pair.
linear the shape of a molecule with sp hybrid orbitals; an alkyne.
Markovnikov rule states that the positive part of a reagent (a hydrogen atom, for example) adds to the carbon of the double bond that already has more hydrogen atoms attached to it. The negative part adds to the other carbon of the double bond. Such an arrangement leads to the formation of the more stable carbocation over other less-stable intermediates.
mass number the total number of protons and neutrons in an atom.
mechanism the series of steps that reactants go through during their conversion into products.
methylene group a —CH2 group.
molecular orbital an orbital formed by the linear combination of two atomic orbitals.
molecule a covalently bonded collection of atoms that has no electrostatic charge.
multiple bond a double or triple bond; multiple bonds involve the atomic p orbitals in side-to-side overlap, preventing rotation.
neutralization the reaction of an acid and a base. The products of an acid and base reaction are a salt and water.
neutron an uncharged particle in the atomic nucleus that has the same weight as a proton. Additional neutrons do not change an element but convert it to one of its isotopic forms.
node a region of zero electron density in an orbital; a point of zero amplitude in a wave.
nonbenzenoid aromatic ring an aromatic ring system that does not contain a benzene ring.
nonbonding electrons valence electrons that are not used for covalent bond formation.
nonterminal alkyne an alkyne in which the triple bond is located somewhere other than the 1 position.
nuclear magnetic resonance spectroscopy a method for measuring how much energy odd-numbered nuclei absorb in the radio frequency range when the atom is exposed to strong magnetic fields. This type of spectroscopy gives information on the environment surrounding the specific nucleus.
nucleofuge see leaving group.
nucleophile a species that is capable of donating a pair of electrons to a nucleus.
nucleophilic substitution a reaction in which a group on a carbon atom, which has a full or partial positive charge, is displaced by a nucleophile.
nucleus the central core of an atom; the location of the protons and neutrons.
optical activity the ability of some chemicals to rotate plane-polarized light.
orbit an area around an atomic nucleus where there is a high probability of finding an electron; also called a shell. An orbit is divided into orbitals, or subshells.
orbital an area in an orbit where there is a high probability of finding an electron; a subshell. All of the orbitals in an orbit have the same principal and angular quantum numbers.
outer-shell electron see valence electrons.
overlap region the region in space where atomic or molecular orbitals overlap, creating an area of high-electron density.
oxidation the loss of electrons by an atom in a covalent bond. In organic reactions, this occurs when a compound accepts additional oxygen atoms.
oxonium ion a positively charged oxygen atom.
ozonide a compound formed by the addition of ozone to a double bond.
ozonolysis the cleavage of double and triple bonds by ozone, O3.
paired spin the spinning in opposite directions of the two electrons in a bonding orbital.
parent name the root name of a molecule according to the IUPAC nomenclature rules; for example, hexane is the parent name in trans-1,2-dibromocyclohexane.
peroxide a compound that contains an oxygen-oxygen single covalent bond.
peroxyacid an acid of general form
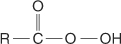
π (pi) bond a bond formed by the side-to-side overlap of atomic p orbitals. A π bond is weaker than a σ bond because of poor orbital overlap caused by nuclear repulsion. Unsaturated molecules are created by π bonds.
π complex an intermediate formed when a cation is attracted to the high electron density of a π bond.
π molecular orbital a molecular orbital created by the side-to-side overlap of atomic p orbitals.
polar covalent bond a bond in which the shared electrons are not equally available in the overlap region, leading to the formation of partially positive and partially negative ends on the molecule.
polarity the asymmetrical distribution of electrons in a molecule, leading to positive and negative ends on the molecule.
precursor the substance from which another compound is formed.
preparation a reaction in which a desired chemical is produced; for example, the dehydration of an alcohol is a preparation for an alkene.
primary carbocation a carbocation to which one alkyl group is bonded.
primary (1°) carbon a carbon atom that is attached to one other carbon atom.
product the substance that forms when reactants combine in a reaction.
propagation step the step in a free radical reaction in which both a product and energy are produced. The energy keeps the reaction going.
protecting group a group that is formed on a molecule by the reaction of a reagent with a substituent on the molecule. The resulting group is less sensitive to further reaction than the original group, but it must be able to be easily reconverted to the original group.
proton a positively charged particle in the nucleus of an atom.
protonation the addition of a proton (a hydrogen ion) to a molecule.
pure covalent bond a bond in which the shared electrons are equally available to both bonded atoms.
pyrolysis the application of high temperatures to a compound.
racemate another name for racemic mixture.
racemic mixture a 1:1 mixture of enantiomers.
rate-determining step the step in a reaction's mechanism that requires the highest activation energy and is therefore the slowest.
rate of reaction the speed with which a reaction proceeds.
reactant a starting material.
reaction energy the difference between the energy of the reactants and that of the products.
reagent the chemicals that ordinarily produce reaction products.
rearrangement reaction a reaction that causes the skeletal structure of the reactant to undergo change in converting to the product.
reduction the gaining of electrons by an atom or molecule. In organic compounds, a reduction is an increase in the number of hydrogen atoms in a molecule.
resonance the process by which a substituent either removes electrons from or gives electrons to a π bond in a molecule; a delocalization of electrical charge in a molecule.
resonance energy the difference in energy between the calculated energy content of a resonance structure and the actual energy content of the hybrid structure.
resonance hybrid the actual structure of a molecule that shows resonance. A resonance hybrid possesses the characteristics of all possible drawn structures (and consequently cannot be drawn). It is lower in energy than any structure that can be drawn for the molecule and thus more stable than any of them.
resonance structures various intermediate structures of one molecule that differ from each other only in the positions of their electrons. None of the drawn resonance structures is correct, and the best representation is a hybrid of all the drawn structures.
R group see alkyl group.
ring structure a molecule in which the end atoms have bonded, forming a ring rather than a straight chain.
rotation the ability of carbon atoms attached by single bonds to freely turn, which gives the molecule an infinite number of conformations.
saturated compound a compound containing all single bonds.
saturation the condition of a molecule containing the most atoms possible; a molecule made up of single bonds.
secondary carbocation a carbocation to which two alkyl groups are bonded.
secondary (2°) carbon a carbon atom that is directly attached to two other carbon atoms.
separation technique a process by which products are isolated from each other and from impurities.
shielding an effect, in NMR spectroscopy, caused by the movement of σ and π electrons within the molecule. Shielding causes chemical shifts to appear at higher magnetic fields (upfield).
σ (sigma) antibonding molecular orbital a σ molecular orbital in which one or more of the electrons are less stable than when localized in the isolated atomic orbitals from which the molecular orbital was formed.
σ (sigma) bond a bond formed by the linear combination of orbitals in such a way that the maximum electron density is along a line joining the two nuclei of the atoms.
σ (sigma) bonding molecular orbital a σ molecular orbital in which the electrons are more stable than when they are localized in the isolated atomic orbitals from which the molecular orbital was formed.
skeletal structure the carbon backbone of a molecule.
SN1 a substitution reaction mechanism in which the slow step is a self ionization of a molecule to form a carbocation. Thus, the rate controlling step is unimolecular.
SN2 a substitution reaction mechanism in which the rate controlling step is a simultaneous attack by a nucleophile and a departure of a leaving group from a molecule. Thus, the rate controlling step is bimolecular.
sp hybrid orbital a molecular orbital created by the combination of wave functions of an s and a p orbital.
sp2 hybrid orbital a molecular orbital created by the combination of wave functions of an s and two p orbitals.
sp3 hybrid orbital a molecular orbital created by the combination of wave functions of an s and three p orbitals.
spin-spin splitting the splitting of NMR signals caused by the coupling of nuclear spins on neighboring nonequivalent hydrogens.
steric hindrance the ability of bulky groups on carbon atoms to prevent or restrict a reagent from reaching a reaction site.
straight-chain alkane a saturated hydrocarbon that has no carbon-containing side chains.
structural isomer also known as a constitutional isomer, structural isomers have the same molecular formula but different bonding arrangements among their atoms. For example, C4H10 can be butane or 2-methylpropane, and C4H8 can be 1-butene or 2-butene.
subatomic particles a component of an atom; either a proton, neutron, or electron.
substituent group any atom or group that replaces a hydrogen atom on a hydrocarbon.
substitution the replacement of an atom or group bonded to a carbon atom with a second atom or group.
substitution reaction a reaction in which one group replaces another on a molecule.
tautomers structural isomers that easily interconvert.
terminal alkyne an alkyne whose triple bond is located between the first and second carbon atoms of the chain.
terminal carbon the carbon atom on the end a carbon chain.
termination step the step in a reaction mechanism that ends the reaction, often a reaction between two free radicals.
tertiary carbocation a carbocation to which three alkyl groups are bonded.
tertiary (3°) carbon a carbon atom that is directly attached to three other carbon atoms.
tetrahaloalkane an alkane that contains four halogen atoms on the carbon chain. The halogen atoms can be located on vicinal or nonvicinal carbon atoms.
thermodynamically controlled reaction a reaction in which conditions permit two or more products to form. The products are in an equilibrium condition, allowing the more stable product to predominate.
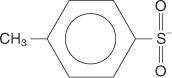
tosyl group a p-toluenesulfonate group:
trigonal planar the shape of a molecule with an sp2 hybrid orbital. In this arrangement, the σ bonds are located in a single plane separated by 60° angles.
triple bond a multiple bond composed of one σ bond and two π bonds. Rotation is not possible around a triple bond. Hydrocarbons that contain triple bonds are called alkynes.
ultraviolet spectroscopy a spectroscopy that measures how much energy a molecule absorbs in the ultraviolet region of the spectrum.
unsaturated compound a compound that contains one or more multiple bonds; for example, alkenes and alkynes.
unsaturation refers to a molecule containing less than the maximum number of single bonds possible because of the presence of multiple bonds.
valence electrons the outermost electrons of an atom. The valence electrons of the carbon atom occupy the 2s, 2px, and 2py orbitals, for example.
valence shell the outermost electron orbit.
vinyl alcohol CH2==CH—OH
vinyl group the CH2==CH— group.
Wurtz reaction the coupling of two alkyl halide molecules to form an alkane.
X group "X" is often used as the abbreviation for a halogen substituent in the structural formula of an organic molecule.
ylide a neutral molecule in which two oppositely charged atoms are directly bonded to each other.
Zaitsev's rule states that the major product in the formation of alkenes by elimination reactions will be the more highly substituted alkene, or the alkene with more substituents on the carbon atoms of the double bond.