Nuclear Magnetic Resonance (NMR) Spectra
Nuclei of atoms with an odd number of protons or neutrons have permanent magnetic moments and quantized nuclear spin states. This means that these types of atoms behave as though they are small magnets spinning on an axis. Placing these types of atoms in a very strong magnetic field separates them into two groups: those that align with the applied field—the field created by the electromagnet of the instrument—and those that align against the applied field.
Aligning against the applied field takes more energy than aligning with the applied field. When the sample is irradiated with radio waves, energy is quantitatively absorbed by the odd‐numbered nuclei, and those aligned with the field will flip to align against the field. Depending upon the environment in which the proton is located, slightly more or less energy is necessary to create the flip. Thus, radio waves of varying frequencies are needed.
Deshielded and shielded protons
In practice, it is easier to fix the radio wave frequency and vary the applied magnetic field than it is to vary the radio wave frequency. The magnetic field “felt” by a hydrogen atom is composed of both applied and induced fields. The induced field is a field created by the electrons in the bond to the hydrogen and the electrons in nearby π bonds. When the two fields reinforce each other, a smaller applied field is required to flip the proton. In this situation, a proton is said to be deshielded. When the applied and induced fields oppose each other, a stronger field must be applied to flip the proton. In this state, the proton is shielded.
The following generalizations apply to shielding and deshielding of the protons in a molecule:
- Electronegative atoms such as nitrogen, oxygen, and halogens deshield hydrogens. The extent of deshielding is proportional to the electronegativity of the hetero atom and its proximity to the hydrogen.
- Electrons on an aromatic ring, double bonded atoms, and triple bonded atoms deshield attached hydrogens.
- A carbonyl group deshields hydrogens on adjacent chains.
- Benzylic and allylic hydrogens are deshielded.
- Electropositive atoms, such as silicon, shield hydrogens.
- Hydrogens attached to a cyclopropane ring and those situated in the π cloud of an aromatic system are strongly shielded.
Chemical shifts
Changes in energy needed to flip protons are called chemical shifts. The location of chemical shifts (peaks) on a NMR spectrum are measured from a reference point that the hydrogens in a standard reference compound—(CH 3) 4Si or tetramethylsilane (TMS)—produce. The amount of energy necessary to flip protons in TMS is assigned the arbitrary value of zero δ. Chemical shifts are measured in parts per million magnetic field strength difference (δ‐scale), relative to TMS.
Deshielded protons absorb downfield on the NMR spectrum (at a lower magnetic field strength than shielded protons).
Mapping nonequivalent hydrogens
Every nonequivalent hydrogen has a unique and characteristic chemical shift that gives rise to a distinct peak or group of peaks. For example, in the propane molecule, two types of nonequivalent hydrogens exist. The first type is methyl hydrogens and the second type is methylene hydrogens. In the following diagram, methyl hydrogens are designated H a while methylene hydrogens are designated H b.

In the propene molecule, four types of nonequivalent hydrogens are designated a through d.

The H c and H d differ because H c is cis to the H b hydrogens while H d is trans.
For the benzene ring system, all hydrogens are equivalent.

Monosubstituted benzenes, however, have nonequivalent hydrogens.

This nonequivalence is due to changing environments as the hydrogens move further away from the electronegative bromine.
Peak areas
The area under a peak is directly proportional to the number of equivalent hydrogens giving rise to the signal.
Peak splitting: Spin-spin coupling
Most chemical shifts aren't single peaks but rather groups or clusters of peaks. These groups and clusters gather because of spin‐spin coupling, which results from the magnetic fields of hydrogen atoms on adjacent carbon atoms reinforcing or opposing the applied magnetic field on an individual proton. In the molecule

the chemical shift for the H a atom is split into three peaks (a triplet), while the chemical shift for the H b atoms is split into two peaks (a doublet).
The general rule for splitting is that the number of peaks created from a chemical shift is calculated as n + 1, where n equals the number of equivalent hydrogen atoms on the adjacent carbon atom(s) that cause the splitting. Applying this rule to the previous compound shows that the carbon adjacent to the carbon bearing the H a hydrogen has two equivalent (H b) hydrogens attached to it. Thus, the H a hydrogen's chemical shift will be split into 2 + 1, or 3, peaks. The chemical shift for the H b hydrogen atoms will be split by the single H a hydrogen on the adjacent carbon into 1 + 1, or 2, peaks. Because the doublet represents the two H b protons and the triplet represents the single H a proton, the areas under the peaks are in a ratio of 2:1 (doublet : triplet ratio).
Coupling constants
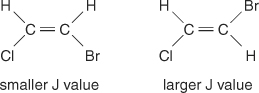
The center‐line spacing between peaks in a cluster—the space from the middle of one peak in a set to the middle of a second peak in that set–caused by spin‐spin coupling is always constant. This constant value is called the coupling constant (J) and is expressed in hertz. The J value depends upon the structural relationship among the coupled hydrogens and is often used to help create a possible structural formula. For example, look at the following isomeric structures of the C 2H 2BrCl (bromochloroethene) compound. In any ethylene or any pair of geometric isomers, the J value will always be larger in trans arrangements than in cis arrangements. In addition, the J values will vary in a regular manner with respect to the electronegativity of the substituents.