In a mass spectrometer, a solid or liquid is heated under reduced pressure to convert it into a gas. The molecules of the gas are then exposed to high‐energy electrons. Collisions between the gaseous molecules and the electrons convert some of the molecules into positively charged ions. These ions are passed through magnetic and electrical fields that separate them into a spectrum based on their mass‐to‐charge ratio (m/z). After separation, the ions arrive at a detector that is calibrated to receive only ions of a plus one charge. The spectrum that results gives the molecular weights of the ions.
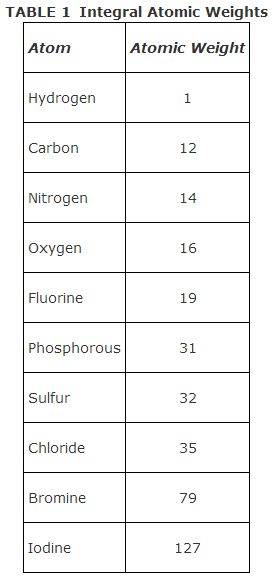
The most important information obtainable from a mass spectrum is the integral molecular weight of the compound. The integral molecular weight is the sum of the light isotopes of the atoms that make up the molecule. The integral masses of atoms used to calculate integral molecular weights are listed below in Table 1
.
The molecular weight of the compound appears as the molecular ion peak on the spectrum. Ordinarily, the molecular ion peak is followed in the spectrum by two peaks; one of m/z one mass unit greater than the molecular ion and a second peak of m/z two mass units greater than the molecular ion. These peaks are called the molecular ion + 1 and the molecular ion + 2 peaks. The intensity of the molecular ion, molecular ion + 1, and molecular ion + 2 peaks exist in an approximate ratio of 100:10:1 for many hydrocarbons or molecules possessing one or more hydrocarbon chains.
The appearance of this type of peak arrangement at the high end of the m/z scale on the mass spectrum is characteristic of the molecular ion. The two higher mass peaks are due to the presence of isotopes of the compound's atoms. The ratio found in the peak pattern is the result of natural isotope abundance of the atoms in the molecule. In a number of spectra, no molecular ion can be found, because the molecular ion is usually very unstable and decomposes into fragment ions before it can reach the detector.
In addition to forming molecular ions, organic molecules decompose into fragment ions in a mass spectrometer. As a result, a host of ions form that have a m/z less than that of the molecular ion. These ions give rise to the characteristic mass spectrum of the molecule. The fragmentation pattern of a molecule is characteristic of the molecule, and an unknown compound may be identified by comparison with a catalog of standard spectra.
Fragment ions are formed through both simple cleavage of larger ions and rearrangements of the molecular ion. Simple cleavage of the molecular ion tends to form stable cations. Thus, simple cleavage generally occurs next to highly branched carbons, at allylic and benzylic bonds, and at carbons adjacent to oxygen and nitrogen atoms.
Fragmentation can also occur by the expulsion of a neutral molecule during the rearrangement of the molecular ion. Many rearrangements involve the loss of small molecules, such as water, carbon monoxide, carbon dioxide, and ammonia.