Ethers are commonly named by listing the names of the groups attached to the oxygen atom and adding the word ether. Examples include:
IUPAC nomenclature names ethers as alkoxy alkanes, alkoxy alkenes, or alkoxy alkynes. The group in the chain that has the greatest number of carbon atoms is designated the parent compound. In the case of aromatic ethers, the benzene ring is the parent compound.
Cyclic ethers, oxygen‐containing ring systems, are normally called by their common names.
The bonds between the oxygen atom and the carbon atoms of the alkyl groups in an ether molecule are polarized due to a difference in electronegativities between carbon and oxygen. In addition, the bond angle between the alkyl groups on the oxygen is 110°. These facts show that ether molecules must be dipoles (molecules having both a center of positive and negative charge) with weak polarities. Thus, the structure of ether is similar to that of water.

However, in water the hydrogen atoms have a greater partial positive charge than the hydrogen atoms on ether. In water, the charge is localized (only on) the hydrogens and not delocalized (spread throughout) as with the alkyl groups, so the charge is stronger in water than in ethers.
Like water, ether is capable of forming hydrogen bonds. However, because of the delocalized nature of the positive charge on the ether molecule's hydrogen atoms, the hydrogens cannot partake in hydrogen bonding. Thus, ethers only form hydrogen bonds to other molecules that have hydrogen atoms with strong partial positive charges. Therefore, ether molecules cannot form hydrogen bonds with other ether molecules. This leads to the high volatility of ethers. Ethers are capable, however, of forming hydrogen bonds to water, which accounts for the good solubility of low molecular weight ethers in water.
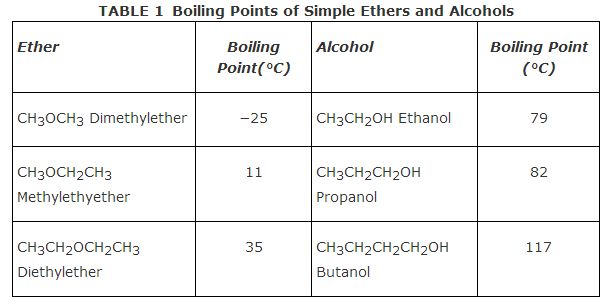
Table shows boiling points for some simple ethers and the boiling points of alcohols of the same number of carbon atoms. Notice that due to the hydrogen bonding between alcohol molecules, all alcohols have appreciably higher boiling points than their isomeric ethers.
|
|
|