Introduction to Alkyl Halides
An alkyl halide is another name for a halogen‐substituted alkane. The carbon atom, which is bonded to the halogen atom, has sp 3 hybridized bonding orbitals and exhibits a tetrahedral shape. Due to electronegativity differences between the carbon and halogen atoms, the σ covalent bond between these atoms is polarized, with the carbon atom becoming slightly positive and the halogen atom partially negative. Halogen atoms increase in size and decrease in electronegativity going down the family in the periodic table. Therefore, the bond length between carbon and halogen becomes longer and less polar as the halogen atom changes from fluorine to iodine.
Physical properties
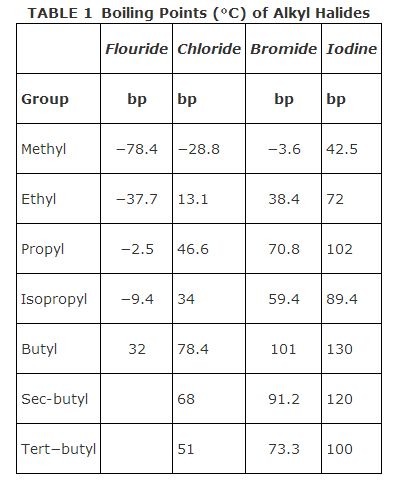
Alkyl halides have little solubility in water but good solubility with nonpolar solvents, such as hexane. Many of the low molecular weight alkyl halides are used as solvents in reactions that involve nonpolar reactants, such as bromine. The boiling points of different alkyl halides containing the same halogen increase with increasing chain length. For a given chain length, the boiling point increases as the halogen is changed from fluorine to iodine. For isomers of the same compound, the compound with the more highly‐branched alkyl group normally has the lowest boiling point. Table summarizes data for some representative alkyl halides.
Alkyl halides are named using the IUPAC rules for alkanes. Naming the alkyl group attached to the halogen and adding the inorganic halide name for the halogen atom creates common names.
