Over 95 percent of the dry weight of a flowering plant is made up of three elements—carbon, hydrogen, and oxygen—taken from the air and water. The remaining 5 percent of the dry weight comes from chemicals absorbed from the soil. Roots absorb the chemicals present in their surroundings, but only 14 of the elements absorbed are necessary for plant growth. These 14 elements, along with carbon, hydrogen, and oxygen, are called the 17 essential inorganic nutrients, or elements. Some of the essentials are needed in larger amounts than others and are called the macronutrients; those needed in lesser amounts are the micronutrients. All elements are needed in specific amounts. Note that there is a dispute among plant physiologists concerning the role of nickel in plant nutrition. Since many physiologists exclude it as essential, in some textbooks, lists like the following consist of only 16 essential inorganic nutrients. The 17 are:
- Macronutrients absorbed from the air: oxygen, carbon, and hydrogen.
- Macronutrients absorbed from the soil: nitrogen, potassium, magnesium, phosphorus, calcium, and sulfur.
- Micronutrients from the soil: iron, boron, chlorine, manganese, zinc, copper, molybdenum, and nickel.
An element is essential if it: 1.) is required for normal growth and reproduction; 2.) can not be replaced by another element; 3.) can be shown to be part of a molecule clearly essential to the plant structure or metabolism.
Plants use elements in differing amounts and forms, some as cations, others as anions. Almost all elements are used in a variety of ways, such as as catalysts for enzymatic reactions (either as part of the enzyme structure or as regulators or activators), as regulators of the movement of water in or out of the cell and maintenance of turgor pressure, as regulators of membrane permeability, as structural components of the cell or of electron receptors in the electron transport system, or as buffers (which maintain the pH within cells).
Two‐thirds of all the naturally occurring chemical elements have been found in plants. Some odd kinds are known to be used metabolically by particular species, but others with no known function are accumulated apparently because they are present in the soil from which the plant is extracting water and ions. These non‐useful chemicals are sequestered in cell vacuoles, as crystals, or as non‐soluble compounds and remain in the plant throughout its life. Plants, therefore, can be useful in locating deposits of minerals, e.g. gold or uranium, and have been used by modern prospectors who collect the vegetation from a site and run spectroscopic analyses on the tissues. Some plants grow only in soils in which a particular element is present and are said to be indicator plants of that element.
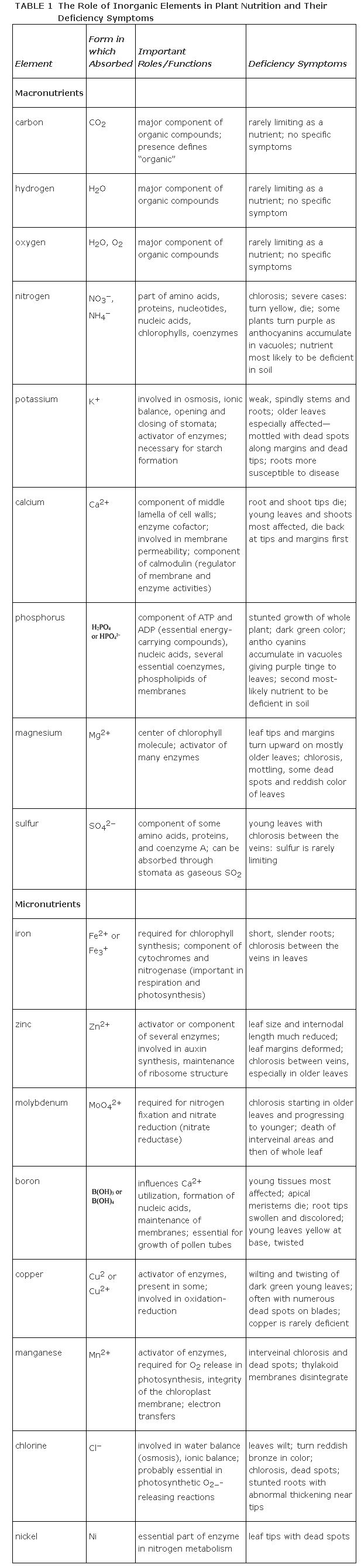
Table highlights the roles of the essential elements in plant nutrition.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|